Superatoms are clusters of atoms that act like a single atom.[1-3] They have unique properties, including diverse functionalization, redox activity, and magnetic ordering. Materials made up of superatoms, so-called clusterassembled solids, hold the promise of high tunability, atomic precision, and robust architectures. Superalkalis are a class of superatoms that have extremely low ionization energy and might serve as reducing agents.[4-6] In this project, we will design new superalkalis and examine their chemical applications.
Celina Sikorska
Earth-abundant nitrogen (N2) is a cheap, nontoxic, and abundant nitrogenous feedstock. The Haber-Bosch process is the predominant source of the world’s ammonia (NH3) production (of 175 million metric tons) and represents more than 90% of the annual production. Despite significant efforts in optimizing the process, it still consumes 1 to 2% of the worldwide annual energy for the high-working temperatures (at 573-873 K) and pressures (at 100-360 atm), currently producing more than 1.6% of global CO2 emissions. So, developing efficient catalysts that convert N2 into ammonia is vital to reduce the growing energy crisis and global warming. Chemical activation of N2 by catalysts is a crucial step towards producing NH3 efficiently and economically. The high stability of N2 makes the conversion difficult. This is a challenging transformation as N2 has a N≡N triple bond and the activation energy is high (941 kJ/mol). The goal of this project is to design and explore superalkalis for N2 activation and conversion into ammonia.
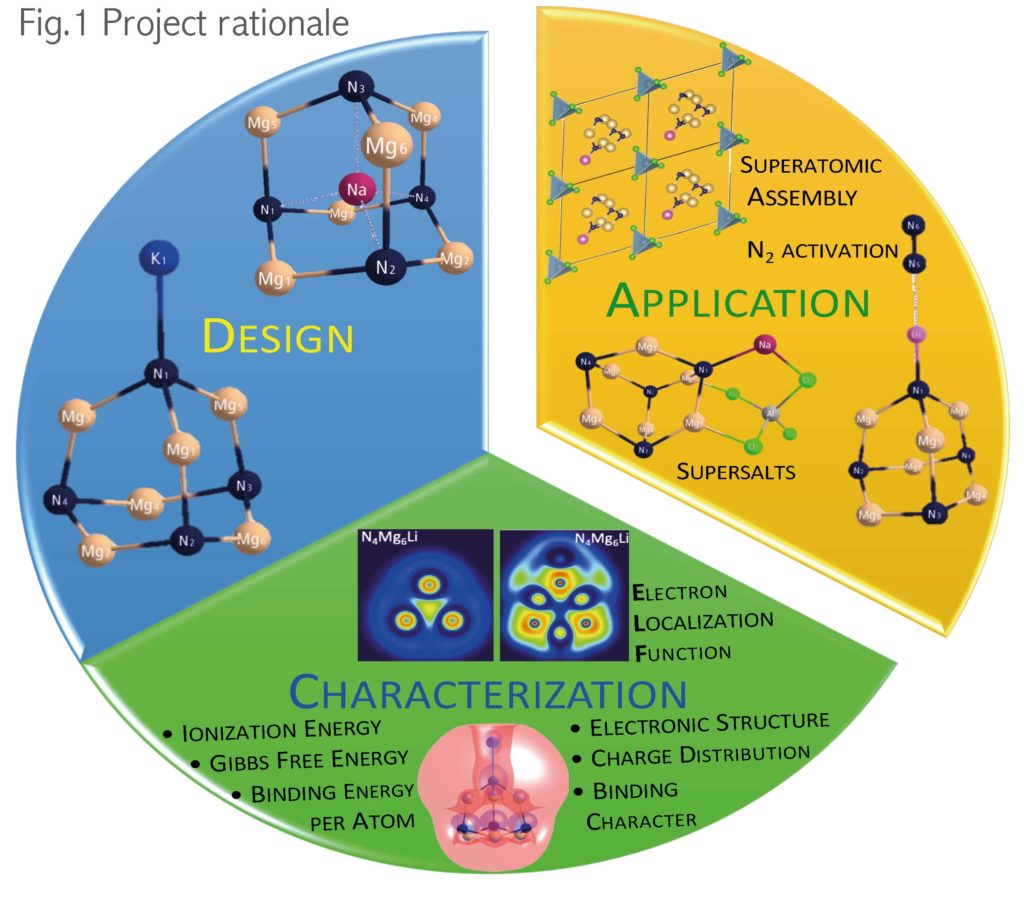
Using a computational (in silico) approach and quantum mechanical techniques, we will realize the following research objectives (Fig.1):
• Design new superalkalis and investigate their physicochemical characterization.
• Design cluster assembled materials and examine their optoelectronic properties.
• Determine the best candidate for N2 reduction reaction and examine the subsequent reactions of activated N2 that lead to its transformation into ammonia (NH3).
We believe that the electronic properties of superalkalis can predict the efficiency of superalkali/N2 complex formation. We will investigate the influence of electronic structure, ionization energy, and geometric structure on the stability and selectivity of superalkali/N2 complexes. The results of this project will enhance our understanding of N2 conversion into ammonia. Subsequently, this can be used to develop a pathway for sustainable nitrogen molecule utilization and fertilizer shortage reduction.
Project details
Project title: Superalkalis as building blocks for the design of unique functional materials and the catalysts for nitrogen conversion into ammonia
Principal Investigator: dr Celina Sikorska
Host institution: University of Gdańsk
Project duration: 01.03.2023 – 28.02.2025
Project’s website: www.etoh.chem.ug.edu.pl/Polonez2023/

References:
[1] P. Jena and Q. Sun, Super Atomic Clusters: Design Rules and Potential for Building Blocks of Materials, Chem Rev 2018, 118, 5755-5870.
[2] K. Hirata, R. Tomihara, K. Kim, K. Koyasu and T. Tsukuda, Characterization of chemically modified gold and silver clusters in gas phase, Phys Chem Chem Phys 2019, 21, 17463-17474.
[3] W. Xie, F. Yu, X. Wu, Z. Liu, Q. Yan and Z. Wang, Constructing the bonding interactions between endohedral metallofullerene superatoms by embedded atomic regulation, Phys Chem Chem Phys 2021, 23, 15899-15903.
[4] W. M. Sun, X. L. Zhang, K. Y. Pan, J. H. Chen, D. Wu, C. Y. Li, Y. Li and Z. R. Li, On the Possibility of Using the Jellium Model as a Guide To Design Bimetallic Superalkali Cations, Chem-Eur J 2019, 25, 4358-4366.
[5] T. S. Zhao, Q. Wang and P. Jena, Rational design of super-alkalis and their role in CO2 activation, Nanoscale 2017, 9, 4891-4897.
[6] C. Sikorska and N. Gaston, N4Mg6M (M = Li, Na, K) superalkalis for CO2 activation, J Chem Phys 2020, 153, 144301.
